AN179 antibody recognizes specifically L1CAM by flow cytometry on rhabdomyosarcoma cell lines
DOI:
https://doi.org/10.24450/journals/abrep.2025.e2341Keywords:
L1CAM, rhabdomyosarcomaAbstract
L1 cell adhesion molecule (L1CAM) is a relevant target in pediatric tumors, including rhabdomyosarcoma (RMS). We validated the recombinant antibody ABCD_AN179, derived from the CE7 hybridoma, for specific detection of L1CAM by flow cytometry. Using CRISPR/Cas9-engineered L1CAM-knockout RMS cell lines (RD, Rh30), ABCD_AN179 showed clear staining of wild-type cells but no signal in knockouts, confirming high specificity. These results demonstrate that ABCD_AN179 specifically detects human L1CAM by flow cytometry and represents a validated recombinant antibody that supports research and translational studies.
Introduction
The human L1 cell adhesion molecule (L1CAM, UniProt #P32004) is a relevant target in pediatric tumors including rhabdomyosarcoma (RMS) (Timpanaro et al., 2023). The hybridoma CE7 was originally selected from mice immunized with the neuroblastoma cell line IMR-32 . Variable regions of the CE7 hybridoma antibody were sequenced and a chimeric mouse/human CE7 antibody (chCE7) was generated (Amstutz et al., 1993). chCE7 target was later identified as the L1CAM protein (Meli et al., 1999). The epitope recognized by this antibody has not been conclusively identified, but evidence points out to a role of glycosylation (Meli et al., 1999; Novak-Hofer et al., 1994) and indicates it to be a tumor-restricted epitope (Hong et al., 2014). Here, we tested if the recombinant antibody ABCD_AN179, derived from the CE7 hybridoma, binds to L1CAM on RMS cell lines by flow cytometry. Results showed a clear staining of wild-type cells but no signal in CRISPR/Cas9 engineered knockout cells, confirming the specificity of this antibody for human L1CAM.
Materials & Methods
Antibodies: ABCD_AN179 (AN179) (ABCD nomenclature, http://web.expasy.org/abcd/) was produced by the Geneva Antibody Facility (http://unige.ch/medecine/antibodies/) as a recombinant mini-antibody consisting of a scFv fused to a rabbit IgG Fc. The scFv heavy and light variable regions from the CE7 hybridoma antibody were joined by a (GGGGS)₃ peptide linker (Amstutz et al., 1993). HEK293 suspension cells, cultured in HEK TF medium (Xell #861-0001, Sartorius) supplemented with 0.1% Pluronic F68 (Sigma #P1300), were transiently transfected with the vector encoding each antibody. Supernatants were collected after 4 days. ABCD_AN179 (60 µg/ml) was aliquoted and stored at -20°C.
Antigen: The antibody was tested against the endogenous L1CAM protein expressed at the surface of human RMS cells RD and Rh30 (RRID: CVCL_1649 and RRID: CVCL_0041). Specificity of the antibody was tested on corresponding L1CAM-knockout single-cell. Briefly, the sgRNA sequence targeting exon 4 of the L1CAM gene (5’-CCTGCTTATCCAGATCCCCG-3’) was designed using the GUIDES online tool (guides.sanjanalab.org, (Meier et al., 2017)). SgRNA sequence was cloned into the pLentiGuide-PURO plasmid (Addgene #52963). The pLentiCRISPR-mCherry vector (Addgene #75161) was used to stably express Cas9 and mCherry in RD and Rh30 cells. 48 h after lentiviral transduction at an MOI of 5, mCherry+ cells were sorted by flow cytometry and cells transiently transfected with pLentiGuide-PURO were selected with 1 µg/ml of puromycin (InvivoGen #ant-pr-1) for 2 days. Cells were then subjected to single-cell culturing by limiting dilution. Expanded clones were validated for L1CAM knockout by western blot and flow cytometry using commercial antibodies (see below). Frameshift-induced indels were confirmed by Sanger sequencing of the targeted region.
Western blotting: For western blot validation of knockout RMS cells, cells were harvested and lysed in RIPA buffer (Thermofisher #89900) supplemented with HaltTM Protease Inhibitor Cocktail (100X, Thermofisher #78430) for 20 min on ice and sonicated for 1 min (10% duty cycle; Branson Sonifier 250). Protein concentrations were determined with the Pierce BCA Protein Assay Kit (Thermofisher #23225) and 30 µg of protein were loaded on an SDS-PAGE gel together with a PageRuler™ Prestained Protein Ladder, 10 to 180 kDa (Thermofisher #26616). Proteins were transfered on a PVDF membrane in 1x transfer buffer (BioRad #1610732. 10x: 250 mM Tris base, 1.92 M glycine, 0.1% SDS) with 10% methanol, at a constant voltage of 100 V at 4 °C for 1.5 h and probed with the commercial anti-L1CAM monoclonal antibody UJ127.11, (Thermofisher #MA1-46044) at a 1:1’000 dilution overnight in TBS-T (TBS 10x: 200 mM Tris (Sigma #1185–53-1), 1.37 M NaCl (Sigma #S9888), pH 7.6; supplemented with Tween 0.1%; (Sigma #P1379)) and 5% skimmed milk. After 1 h incubation at room temperature with the secondary antibody horse anti-mouse IgG coupled to horseradish peroxidase (Cell Signaling #7076) at a dilution 1:10’000, the signal was developed with SuperSignal West Femto Maximum Sensitivity Substrate (Thermofisher #34094) and measured by using ChemiDoc MP Imaging System (Bio-Rad).
Flow cytometry: Cells were detached using accutase treatment (Sigma-Aldrich), washed with PBS, and 250’000 cells were resuspended in 200 µL PBS/2%BSA. After centrifugation (3 min at 400 g at RT), cells were resuspended in 100 µL PBS/2%BSA containing the AN179 antibody at a 0.25 ng/µL concentration. Samples were then incubated 30 min at room temperature with gentle vortexing every 10 min and washed with 400 µL PBS/2%BSA. AN179 tested samples were resuspended in 100 µL of PBS/2%BSA with a goat anti-rabbit secondary antibody conjugated with AlexaFluor-488 (1:1’000 dilution; Thermofisher #A-11034). Samples were then incubated 30 min at room temperature with gentle vortexing every 10 min and washed twice in 500 µL PBS/2%BSA. Washed cells were resuspended in 200 µL PBS/2%BSA, transferred to a transparent flat bottom 96-well plate and analyzed with a CytoFLEX S Flow Cytometer (AF488 channel, gain 100; Beckman Coulter). Knockout clones were validated by the same protocol using the commercial anti-L1CAM clone REA163|5G3 labeled with R-phycoerythrin (5G3 PE, Miltenyi Biotec #130-100-691) at a 1:100 dilution.
Results & Discussion
CRISPR/Cas9 knockout of L1CAM in RD and Rh30 single clone cells was first validated by western blotting using commercial antibody UJ127.11 (Figure 1A). L1CAM signal could not be detected in RD_1-5 and Rh30_1-2 clones. These two clones were then selected for flow cytometry validation with a PE-labelled commercial monoclonal antibody clone 5G3 (Figure 1B). Sequencing of the targeted region confirmed the presence of frameshift induced deletions (data not shown).
Fig. 1. Validation of L1CAM knockout clones by western blotting and flow cytometry. A. Western blotting of two single cell clones each for RD and Rh30 cells revealed a complete absence of L1CAM specific band around 200 kDa for RD clone 1-5 and Rh30 clone 1-2, in the other clones a faint band was visible when exposure was maximized suggesting incomplete knockout. B. RD clone 1-5 and Rh30 clone 1-2 were then incubated with a highly validated L1CAM antibody (clone 5G3, Miltenyi). Absence of L1CAM on the surface in the selected knockout clones was confirmed.
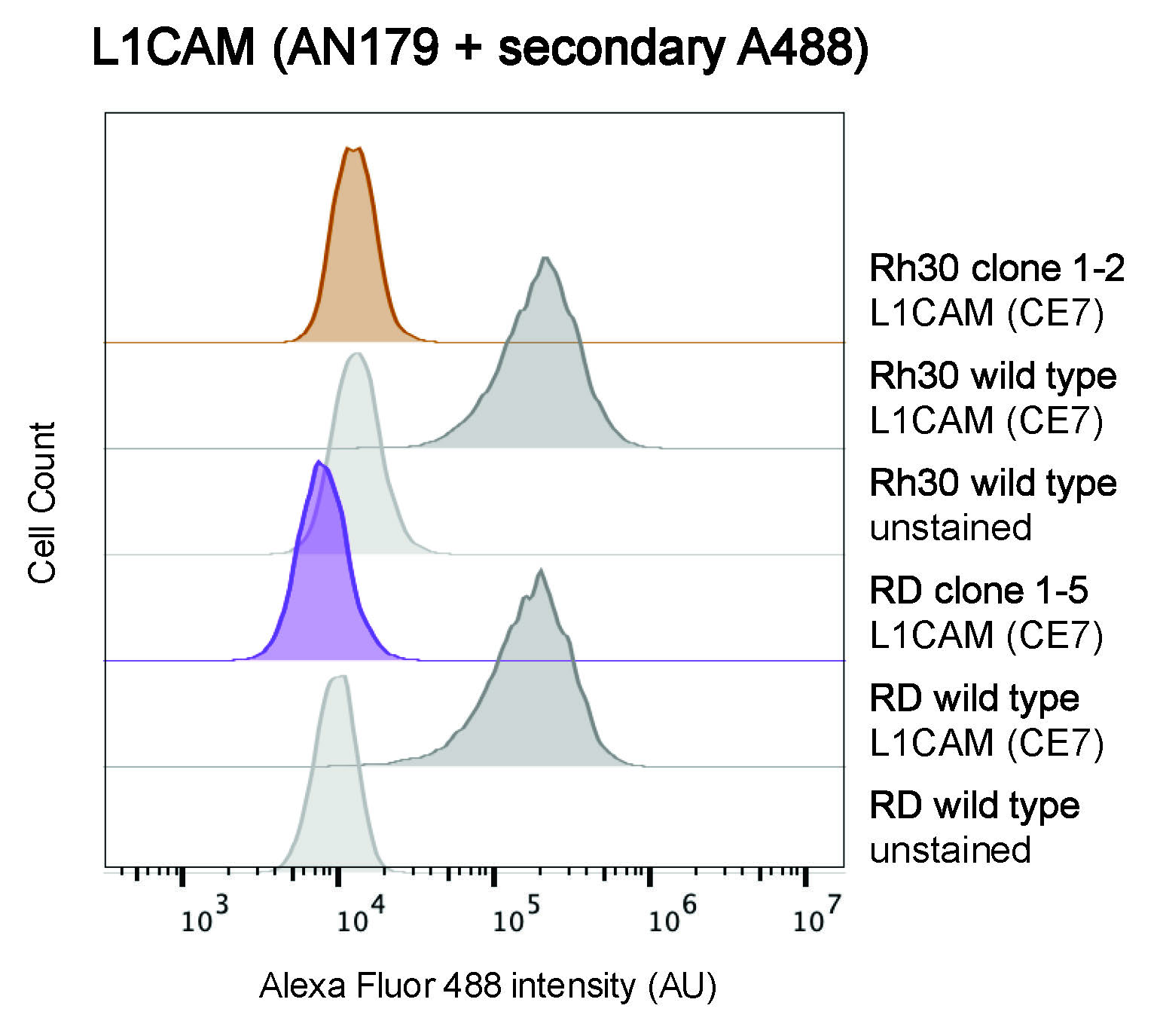
Next, we tested the AN179 antibody using flow cytometry. Different dilutions of the antibody were initially tested (2 ng/mL to 0.25 ng/mL) on RD wild type cells and 0.25 ng/mL was selected as the optimal concentration, since all the concentrations gave a robust signal (data not shown). Wild type and L1CAM knockout RMS cells were incubated with 0.25 ng/mL AN179 antibody and detected with an anti-rabbit secondary labelled with Alexa488 (Figure 2). The AN179 antibody could detect L1CAM on the surface of wild type RD and Rh30 cells. The lack of signal on RD clone 1-5 and Rh30 clone 1-2, demonstrate that the binding observed at the surface of wild type RMS cells is specific to human L1CAM. Our results demonstrate that the antibody AN179 specifically recognizes L1CAM on the surface of RMS cells and that this recombinant antibody can be used for flow cytometry. Currently, the sequence of the CE7 clone is used as antigen binding domain in a Chimeric Antigen Receptor (CAR) constructs testing the use of L1CAM CAR T cells against neuroblastoma in clinical trials and represents a high quality and convenient tool to verify the expression of the epitope targeted by the CE7-based CAR.
Fig. 2. Specific detection of L1CAM by the AN179 antibody on rhabdomyosarcoma cell lines. RMS cells were incubated with 0.25 ng/mL of ABCD_AN179 antibody and after washing, with anti-rabbit-Alexa488 (1:100). A specific signal was detected on wild type RD and Rh30 cells, while no signal was detected on the L1CAM knockout RD clone 1-5 and Rh30 clone 1-2.
Conflict of interest
The authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Amstutz, H., Rytz, C., Novak-Hofer, I., Spycher, M., Schubiger, P. A., Blaser, K., & Morgenthaler, J. J. (1993). Production and characterization of a mouse/human chimeric antibody directed against human neuroblastoma. Int J Cancer, 53(1), 147-152. https://doi.org/ 10.1002/ijc.2910530127
Hong, H., Stastny, M., Brown, C., Chang, W. C., Ostberg, J. R., Forman, S. J., & Jensen, M. C. (2014). Diverse solid tumors expressing a restricted epitope of L1-CAM can be targeted by chimeric antigen receptor redirected T lymphocytes. J Immunother, 37(2), 93-104. https://doi.org/ 10.1097/CJI.0000000000000018
Meier, J. A., Zhang, F., & Sanjana, N. E. (2017). GUIDES: sgRNA design for loss-of-function screens. Nat Methods, 14(9), 831-832. https://doi.org/ 10.1038/nmeth.4423
Meli, M. L., Carrel, F., Waibel, R., Amstutz, H., Crompton, N., Jaussi, R., Moch, H., Schubiger, P. A., & Novak-Hofer, I. (1999). Anti-neuroblastoma antibody chCE7 binds to an isoform of L1-CAM present in renal carcinoma cells. Int J Cancer, 83(3), 401-408. https://doi.org/10.1002/(sici)1097-0215(19991029)83:3<401::aid-ijc17>3.0.co;2-a
Novak-Hofer, I., Amstutz, H. P., Morgenthaler, J. J., & Schubiger, P. A. (1994). Internalization and degradation of monoclonal antibody chCE7 by human neuroblastoma cells. Int J Cancer, 57(3), 427-432. https://doi.org/ 10.1002/ijc.2910570322
Pinto, N., Künkele, A., Albert, C., Taylor, M., Ullom, H., Wilson, A., Huang, W., Wendler, J., Seidel, K., Brown, C., Gustafson, J., Rawlings-Rhea, S., Beebe, A., Mgebroff, S., Gardner, R., Jensen, M., & Park, J. (2024). First-in-human comparison of second- versus third-generation L1CAM-specific CAR T cells in patients with primary refractory or relapsed neuroblastoma. PREPRINT (Version 1) available at Research Square. https://doi.org/ 10.21203/rs.3.rs-3859120/v1
Schonmann, S. M., Iyer, J., Laeng, H., Gerber, H. A., Kaser, H., & Blaser, K. (1986). Production and characterization of monoclonal antibodies against human neuroblastoma. Int J Cancer, 37(2), 255-262. https://doi.org/ 10.1002/ijc.2910370214
Timpanaro, A., Piccand, C., Uldry, A. C., Bode, P. K., Dzhumashev, D., Sala, R., Heller, M., Rossler, J., & Bernasconi, M. (2023). Surfaceome Profiling of Cell Lines and Patient-Derived Xenografts Confirm FGFR4, NCAM1, CD276, and Highlight AGRL2, JAM3, and L1CAM as Surface Targets for Rhabdomyosarcoma. International Journal of Molecular Sciences, 24(3). https://doi.org/ 10.3390/ijms24032601
Downloads
Published
Section
How to Cite
License
Some rights reserved 2025 Caroline Piccand, Michele Bernasconi

This work is licensed under a Creative Commons Attribution 4.0 International License.



