Camelid single-domain antibody ciA-B5 recognizes light chain of botulinum neurotoxin type A by ELISA
DOI:
https://doi.org/10.24450/journals/abrep.2025.e1870Keywords:
ciA-B5, ELISA, Recombinant antibody, VHH, Nanobody, Light chain, Botulin neurotoxinAbstract
Camelid single-domain antibody (VHH) ciA-B5 detects by ELISA the light chain (LC) of the botulinum neurotoxin type A (BoNT/A).
Introduction
Botulinum neurotoxin type A (BoNT/A, UniProt #P0DPI1) poses a great threat to humans due to its most potent toxicity with the longest duration of paralysis. BoNT/A consists of a heavy chain (HC) encompassing the receptor binding domain (HC) and the translocation domain (HN), and a light chain (LC) which is a zinc-dependent endopeptidase, capable of specific cleavage on neuronal SNARE proteins. Previous research has showed that VHH ciA-B5 can bind to the HN domain of the heavy chain of BoNT/A and has demonstrated its ability to neutralize BoNT/A in a mouse model . In the present study, we describe the reactivity in ELISA of this VHH against LC of BoNT/A.
Materials & Methods
Antibodies: The gene coding for ciA-B5 (ABCD_AW306, ABCD nomenclature, http://www.expasy.org/abcd) was codon-optimized for expression in E. coli, synthesized by Genscript, and cloned into pET22b (Novagen #69744-3) expression vector, which was modified to carry a FLAG tag (DYKDDDDK) at the C-terminal end for detection.His-tagged recombinant VHHwas expressed in E. coliBL21(DE3) and purified by Ni-NTA spin columns (Qiagen #31014) following the manufacturer's instructions.
Antigen: The bont/LCA1 fragment (residues 1 - 420 of BoNT/A1) was cloned into pET45b vector with an N-terminal His-tag (Novagen #71327-3). LC/A1was expressed in E. coliRosetta™ 2(DE3) and purified using Ni-NTA spin columns (Qiagen #31014), following the manufacturer's instructions. HC/A1 fragment or receptor-binding domain of BoNT/A1 (residue 871-1296 of BoNT/A1) was produced the same way and used as a negative control.
Protocol: High Bind Stripwell™ Microplates (Corning #07-200-24) were coated with 2 μg/mL recombinant antigens (LC/A1 and HC/A1) in Phosphate Buffer Saline (PBS 1X) at 4°C overnight. Each well was rinsed twice with 300 μl of washing buffer (PBS 1X supplemented with 0.05% (w/v) Tween-20), then blocked for 1 hour with 300 µl of PBS 1X supplemented with 1% (w/v) BSA. After washing, wells were incubated for 2 hours at 37oC with 100 µl of the recombinant VHH diluted in PBS 1X with 0.5% (w/v) BSA. The wells were washed three times with washing buffer, then incubated with anti-DYKDDDDK (FLAG) Antibody Rabbit - HRP Conjugate (Immunology Consultants Lab #RFLG-45P) (dilution 1:10000) for 1 hour at 37oC. After six washes, Tetramethylbenzidine (TMB) substrate (Sigma #T5569) was added (100 μl per well). Reactions were stopped by adding 100 μl of 1 M HCl to each well. The experiment was run in triplicate, the optical density of each sample was analyzed at 450 nm with a reference reading at 630 nm. EC50 values (the half-maximal effective concentration) were calculated via non-linear regression analysis using GraphPad Prism.
Results & Discussion
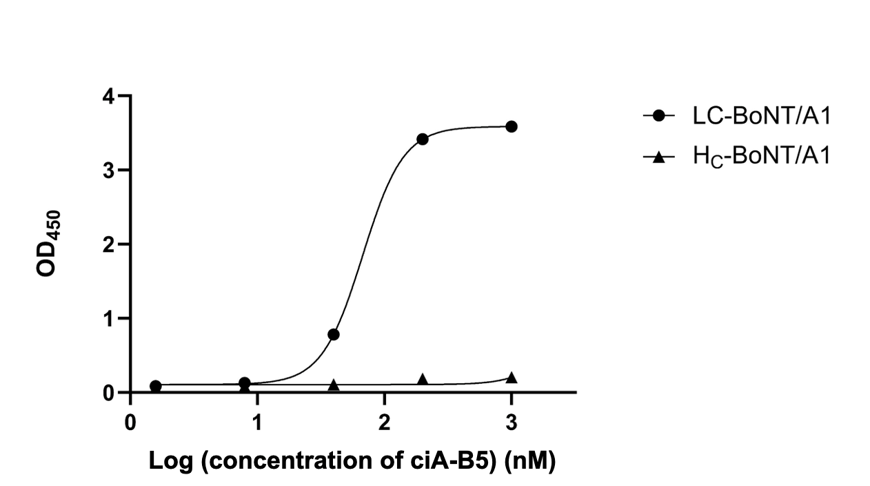
VHH ciA-B5 bound in a concentration-dependent manner to LC/A1 but did not bind to the HC/A1 negative control (Fig.1). From the ELISA dose-response curve, EC₅₀ value was calculated to be approximately 67.3 nM. Of note, ciA-B5 displayed moderate affinity toward LC/A1 comparing to a panel of VHHs (produced in our laboratory) consisting of JPU-A5, JPU-C10 and JPU-D12 from Lam et al. (2020) and A1, A16 from Conway et al. (2010) (data not shown) .In a previous study, ciA-B5 was reported to bind to the HN region of BoNT/A1 This conclusion was based on the crystallization of a hetero-tetrameric complex (LCHN/A–ciA-B5–ciAD-12–ciA-H7) including both LC and HN of BoNT/A1, along with three VHHs (ciA-H7, ciA-D12, ciA-B5), in which ciA-H7 interacts with residues in the long helix α5 (Loop120, 170, and 250), and α10 of LC/A1; ciA-D12 interacts with residues in the C-terminal loop, the N-terminal loop, and α10 of LC/A1; and ciA-B5 interacts to residues 600–616 in HN domain of BoNT/A1. However, this report did not conclusively exclude the possibility that ciA-B5 can bind to the LC/A1. As both ciA-H7 and ciA-D12 interact with numerous regions of LC/A1, it is conceivable that interaction between ciA-B5 and LC/A1 could be obscured in the crystallographic analysis. In the present study, our results clearly demonstrated that ciA-B5 binds to LC/A1 in a concentration-dependent manner in ELISA assay. Therefore, we hypothesized that ciA-B5 can interact with both the LC and HN domains of BoNT/A1.
Figure 1. ELISA dose-response curve of VHH ciA-B5 to LC-BoNT/A1 and HC-BoNT/A1 as negative control.
Acknowledgements
Research reported in this publication was supported by Hanoi University of Science and Technology [Grant number: T2022-TĐ-002]
Conflict of interest
The authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Conway, J. O., Sherwood, L. J., Collazo, M. T., Garza, J. A., & Hayhurst, A. (2010). Llama single domain antibodies specific for the 7 botulinum neurotoxin serotypes as heptaplex immunoreagents. PLoS ONE, 5(1). https://doi.org/10.1371/journal.pone.0008818
Lam, K. H., Tremblay, J. M., Perry, K., Ichtchenko, K., Shoemaker, C. B., & Jin, R. (2022). Probing the structure and function of the protease domain of botulinum neurotoxins using single-domain antibodies. PLoS Pathogens, 18(1). https://doi.org/10.1371/journal.ppat.1010169
Lam, K. ho, Tremblay, J. M., Vazquez-Cintron, E., Perry, K., Ondeck, C., Webb, R. P., McNutt, P. M., Shoemaker, C. B., & Jin, R. (2020). Structural Insights into Rational Design of Single-Domain Antibody-Based Antitoxins against Botulinum Neurotoxins. Cell Reports, 30(8). https://doi.org/10.1016/j.celrep.2020.01.107
Mukherjee, J., Tremblay, J. M., Leysath, C. E., Ofori, K., Baldwin, K., Feng, X., Bedenice, D., Webb, R. P., Wright, P. M., Smith, L. A., Tzipori, S., & Shoemaker, C. B. (2012). A novel strategy for development of recombinant antitoxin therapeutics tested in a mouse botulism model. PLoS ONE, 7(1). https://doi.org/10.1371/journal.pone.0029941
Downloads
Published
Section
How to Cite
License
Some rights reserved 2025 Nga Quynh Pham, Tam Trang Mai, Tran Bao Anh Dang, Anh Minh Nguyen, Anh Thao Nguyen, Anh Phuong Tran, Tran Nhat Minh Dang, Van Khanh Tran, Hoa Quang Le

This work is licensed under a Creative Commons Attribution 4.0 International License.



