The RB824 antibody recognizes the mNeonGreen protein from Amphioxus lanceolatum by ELISA and immunofluorescence
DOI:
https://doi.org/10.24450/journals/abrep.2024.e1529Abstract
RB820, RB821, RB822, RB823, RB824, and RB825 antibodies were assessed for their ability to recognize the Amphioxus lanceolatum mNeonGreen protein fused to a Twin-Strep-Tag using both ELISA and immunofluorescence assays. All the tested antibodies demonstrated the ability to detect the antigen in ELISA. RB824 successfully detected Amphioxus lanceolatum mNeonGreen by immunofluorescence in transiently transfected RAW264.7 cells
Introduction
The mNeonGreen protein (UniProt #A0A1S4NYF2) is a monomeric yellow-green fluorescent protein derived from a tetrameric fluorescent protein found in the cephalochordate Amphioxus lanceolatum (Shaner et al., 2013). This recently developed protein exhibits 3-5 times greater brightness compared to GFP and enables the detection of low expression levels (Hostettler et al., 2017). To further optimize mNeonGreen detection, we investigated the ability of six recombinant antibodies (RB820, RB821, RB822, RB823, RB824, and RB825) to detect a Twin-Strep-Tagged mNeonGreen protein by ELISA. Additionally, we assessed their ability to detect mNeonGreen by immunofluorescence in transiently transfected RAW 264.7 cells using an expression vector encoding mNeonGreen.
Materials & Methods
Antibodies: ABCD_RB820, ABCD_RB821, ABCD_RB822, ABCD_RB823, ABCD_RB824 and ABCD_RB825 antibodies (ABCD nomenclature, http://web.expasy.org/abcd/) were discovered by the Geneva Antibody Facility (http://unige.ch/medecine/antibodies/) and produced as minibodies with the antigen binding VHH fused to a guinea pig IgG Fc (RB820, RB821, RB822, RB823, RB824 and RB825). HEK293 suspension cells growing in HEK TF medium (Xell #861-0001, Sartorius) supplemented with 0.1% Pluronic F68 (Sigma #P1300) were transiently transfected with the vectors coding for each VHH-Fc. Supernatants (~70-120 mg/l) were collected after 5 days.
Antigen: The antibodies were originally raised against the mNeonGreen protein (residues 25-260) fused to a Twin-Strep-Tag (TST). The same construction was used as antigen for ELISA detection. An irrelevant TST tagged peptide from the human tenascin protein (UniProt #P24821, residues 1619-1710) was used as a negative control.
ELISA Protocol: The whole procedure was carried out at room temperature. TST tagged proteins at saturating concentration (10 pmol/well) were incubated on MaxiSorp 96-well plates (Nunc #44-2404-21) for 30 min. Each well was rinsed three times with 100 μl of washing buffer (PBS + 0.5% (w/v) BSA + 0.05% (w/v) Tween20), then incubated for 1 hour with 50 µl of antibody-containing supernatant diluted in washing buffer (Fig. 1). After rinsing 3 times (100 µl washing buffer), wells were incubated with horseradish peroxidase-coupled goat anti-guinea pig IgG (Invitrogen #A18775, dilution 1:1000, 50 μl per well) for 30 min. After 3 rinses, Tetramethylbenzidine (TMB) substrate (Sigma #T5569) was added (50 μl per well). The reaction was stopped by the addition of 25 μl of 2 M H2SO4. The absorbance (OD) was measured at 450 nm, and the absorbance at 570 nm was subtracted.
Cell culture and transient transfection: RAW 264.7 cells, growing in an 8-chamber slide (Nunc Lab-Tek II, #154534) in DMEM medium (ThermoFisher #31966021) supplemented with 10% FBS (PAN Biotech #P40-37500), penicillin and streptomycin (Gibco #15140-122), were transiently transfected (jetPRIME®, Polyplus) with the pCDNA3 vector encoding the mNeonGreen protein or with the empty vector 1 day before immunofluorescence experiments.
Immunofluorescence protocol: Transfected RAW 264.7 cells were fixed in 4% paraformaldehyde before incubation with the primary antibody (RB820, RB821, RB822, RB523, RB824 or RB825, 1:100 in Dako REAL Antibody Diluent (Dako, #S2022)). Cells were then washed twice for 5 min in IHC buffer (0.05M Tris, 0.15M NaCl, 0.05% Tween 20) and incubated with the secondary antibody: Alexa Fluor® 647 AffiniPure™ Goat Anti-Guinea Pig IgG (Jackson ImmunoResearch, #106605003, 1:500 in Dako REAL Antibody Diluent). Cells were then washed twice for 5min in IHC buffer and once for 5 min in PBS and nuclei were stained in blue (DAPI, 1:1000). Finally, cells were washed once for 5 min in PBS, chambers were removed from the slide and FluoreGuard Mounting medium (Scytek #FMH030) added between the slide and a microscope cover glass. Images were acquired with a confocal microscope (Zeiss, LSM700).
Results
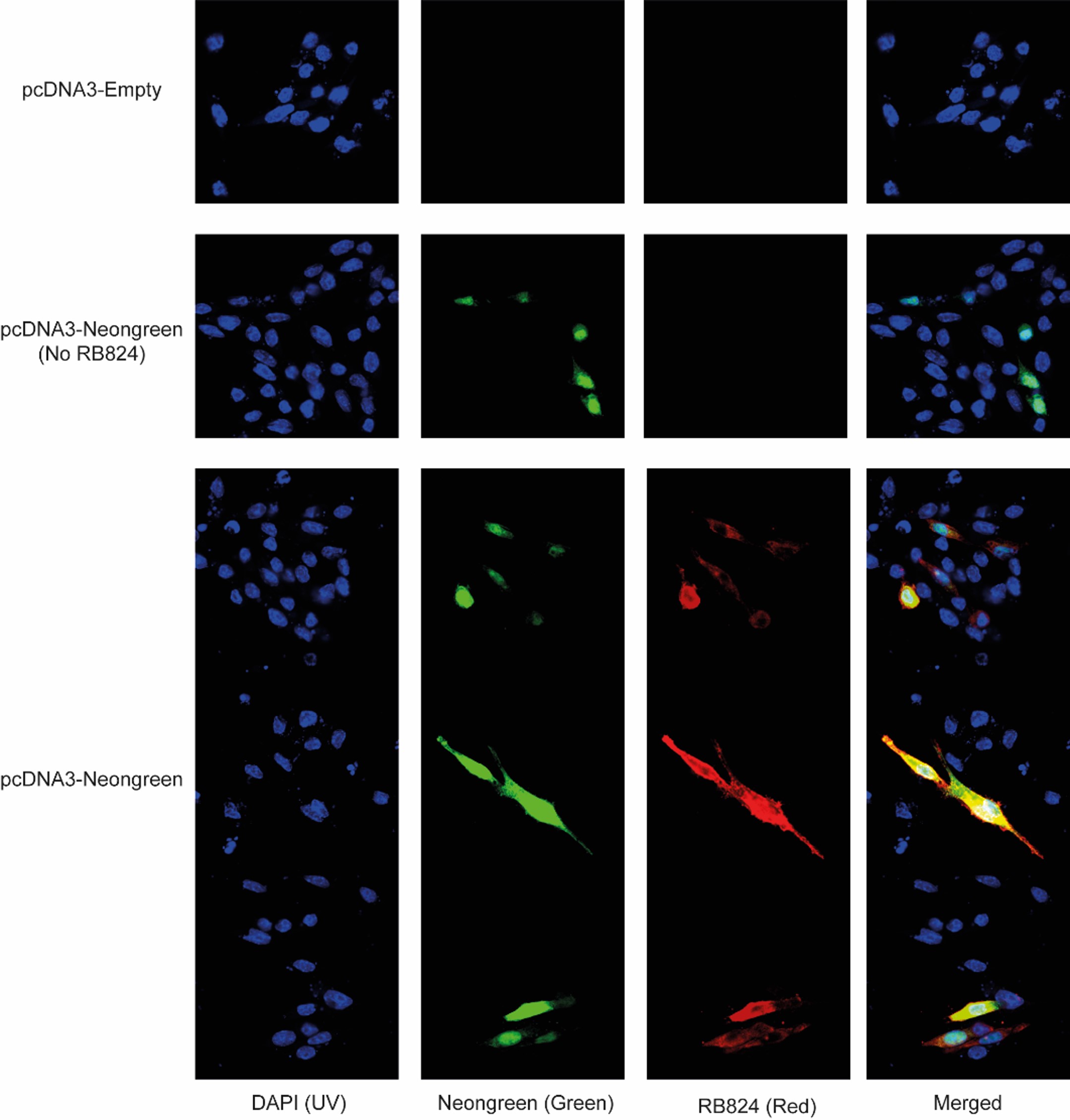
The ELISA results revealed a concentration-dependent binding of antibodies RB820, RB821, RB822, RB823, RB824, and RB825 to the mNeonGreen protein fused to TST (Fig. 1). These antibodies exhibited no binding to the negative control (irrelevant TST tagged peptide). When used for immunofluorescence detection, RB824 exhibited specific recognition of RAW 264.7 cells expressing mNeonGreen (Fig. 2). RB820, RB821, RB822, RB823, and RB825 antibodies displayed a non-detectable immunofluorescence signal in mNeonGreen-expressing RAW 264.7 cells (data not shown).
Figure 1. Specific binding of RB antibodies to the TST tagged mNeonGreen protein but not to an irrelevant TST tagged peptide (shown only for RB820; other background curves are superimposed), as detected by ELISA.
Figure 2. Specific binding of the RB824 antibody to mNeonGreen in RAW 264.7 cells, as detected by immunofluorescence. Living cells transfected with pcDNA-mNeonGreen exhibited green fluorescence, contrasting with their pcDNA-Empty transfected counterparts. RB824 antibodies (labelled in red) detected the presence of mNeonGreen by immunofluorescence. Merged analysis confirmed the colocalization of green and red fluorescence in transfected cells.
Conflict of interest
The authors declare no conflict of interest.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Shaner, N. C., Lambert, G. G., Chammas, A., Ni, Y., Cranfill, P. J., Baird, M. A., Sell, B. R., Allen, J. R., Day, R. N., Israelsson, M., Davidson, M. W., & Wang, J. (2013). A bright monomeric green fluorescent protein derived from Branchiostoma lanceolatum. Nature methods, 10(5), 407–409. PMID: 23524392.
Hostettler, L., Grundy, L., Käser-Pébernard, S., Wicky, C., Schafer, W. R., & Glauser, D. A. (2017). The Bright Fluorescent Protein mNeonGreen Facilitates Protein Expression Analysis In Vivo. G3 (Bethesda, Md.), 7(2), 607–615. PMID: 28108553.
Downloads
Published
Section
How to Cite
License
Some rights reserved 2024 Laurie Vaillant, Emiliana Rodriguez, Assunta Caruso, Sebastien Fauteux-Daniel, Cem Gabay

This work is licensed under a Creative Commons Attribution 4.0 International License.



